Gmp Process Validation Protocol . Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process.
from www.validation-online.net
Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web the overarching text presented in this annex constitutes the general principles of the new guidance on.
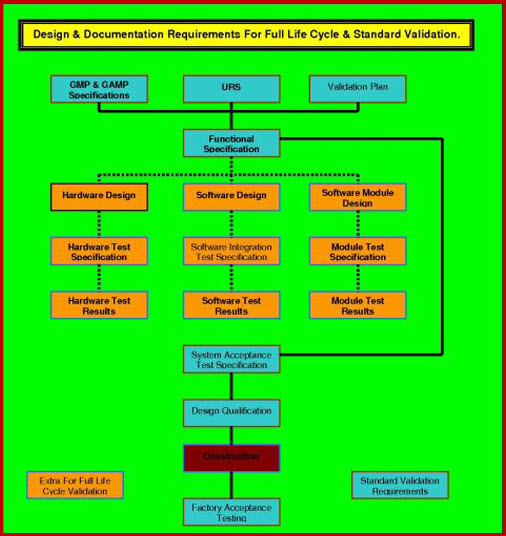
Software Validation FDA EU WHO cGMP QbD FLCV SOP GxP
Gmp Process Validation Protocol Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web process validation can be defined as documented evidence that the process, operated within established parameters,.
From cookinglove.com
Gmp audit checklist pdf Gmp Process Validation Protocol Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web the overarching text presented. Gmp Process Validation Protocol.
From www.gmpsop.com
All infographic embed Codes from Gmp Process Validation Protocol Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this document provides. Gmp Process Validation Protocol.
From www.presentationeze.com
FDA GMP QSR Validation. PresentationEZEPresentationEZE Gmp Process Validation Protocol Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web this document provides guidance on the process validation information and. Gmp Process Validation Protocol.
From www.greenlight.guru
IQ, OQ, PQ A Quick Guide to Process Validation Gmp Process Validation Protocol Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web. Gmp Process Validation Protocol.
From www.getreskilled.com
What's an IQ OQ PQ Validation Protocol & why's it critical in pharma? Gmp Process Validation Protocol Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web. Gmp Process Validation Protocol.
From www.slideserve.com
PPT Validation Part 5 Review and summary PowerPoint Presentation Gmp Process Validation Protocol Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web the guide presents a review of the. Gmp Process Validation Protocol.
From www.pharmaqualification.com
Prospective Process Validation Gmp Process Validation Protocol Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web. Gmp Process Validation Protocol.
From lmgnewyork.com
GMP Good Manufacturing Practice LMG New York Gmp Process Validation Protocol Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web the guide presents a review of the. Gmp Process Validation Protocol.
From www.scribd.com
Basic GMP Checklist for Pharmaceutical Plants Verification And Gmp Process Validation Protocol Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this document provides guidance on the process validation information and. Gmp Process Validation Protocol.
From www.presentationeze.com
Deviation Control in a GMP Process Information & TrainingPresentationEZE Gmp Process Validation Protocol Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web this document provides. Gmp Process Validation Protocol.
From pharmabeginers.com
Analytical Method Transfer (USP 1224) Guideline Pharma Beginners Gmp Process Validation Protocol Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web process validation can be defined as. Gmp Process Validation Protocol.
From www.aplyon.com
Validation Protocols Reports Procedure Gmp Process Validation Protocol Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web process validation is the documented evidence that the process, operated. Gmp Process Validation Protocol.
From isosrilanka.ascentworld.com
GMP Certification Good Manufacturing Practice Ascent Gmp Process Validation Protocol Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,.. Gmp Process Validation Protocol.
From www.presentationeze.com
FDA GMP QSR Equipment and Maintenance PresentationEZE Gmp Process Validation Protocol Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web this document provides guidance on the process validation information and data. Gmp Process Validation Protocol.
From angstromtechnology.com
What Do the GMP Qualification & Validation Processes Look Like Gmp Process Validation Protocol Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the.. Gmp Process Validation Protocol.
From www.validation-online.net
Software Validation FDA EU WHO cGMP QbD FLCV SOP GxP Gmp Process Validation Protocol Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web process validation is the documented evidence that the process, operated within established parameters, can perform. Web process validation can. Gmp Process Validation Protocol.
From www.studypool.com
SOLUTION LPA guidance GMP process validation Studypool Gmp Process Validation Protocol Web process validation can be defined as documented evidence that the process, operated within established parameters,. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web this document provides guidance on the process validation information and data to be provided in regulatory submissions for the. Web the overarching text presented in this annex. Gmp Process Validation Protocol.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Gmp Process Validation Protocol Web the guide presents a review of the types and extent of validations required by gmp, the preparation of a master validation plan,. Web the overarching text presented in this annex constitutes the general principles of the new guidance on. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process. Web process validation is. Gmp Process Validation Protocol.